
Historically, the anode was known as the zincode. Anions tend to move towards the anode where they can undergo oxidation. In an electrolytic cell, the anode is the wire with a positive charge. In both a galvanic cell and an electrolytic cell, the anode is the electrode where oxidation reaction occurs. Therefore, the negatively charged electrons flow out the anode of a galvanic cell, into an external circuit connected to the cell. A common mnemonic is anode current into the device. The direction of current in a circuit is opposite to the direction of electron flow. In contrasts with a cathode, an electrode by which conventional current leaves an electrical device. AnodeĪn anode is an electrode by which the conventional current enters into a polarized electrical device. Items to be plated with pure metal are attached and become part of the cathode in the solution. When metal ions are reduced, they form a pure metal surface on the cathode. Electroplating Metal Cathode (Electrolysis) The anode of the galvanic cell gives off electrons and return from the circuit into the cell through the cathode. This positive pole is connected to allow the circuit to be completed. In a galvanic cell, the cathode is the positive pole. While studying the relative reducing power of two redox agents, the couple for making the more reducing species is more cathodic with respect to the more easily reduced reagent. Some results of reduction at the cathode are pure metal or hydrogen gas from metal ions. In an electrolytic cell, the cathode is where the negative polarity is applied. The cathodic current is the flow of electrons from the cathode interface to a species in solution.
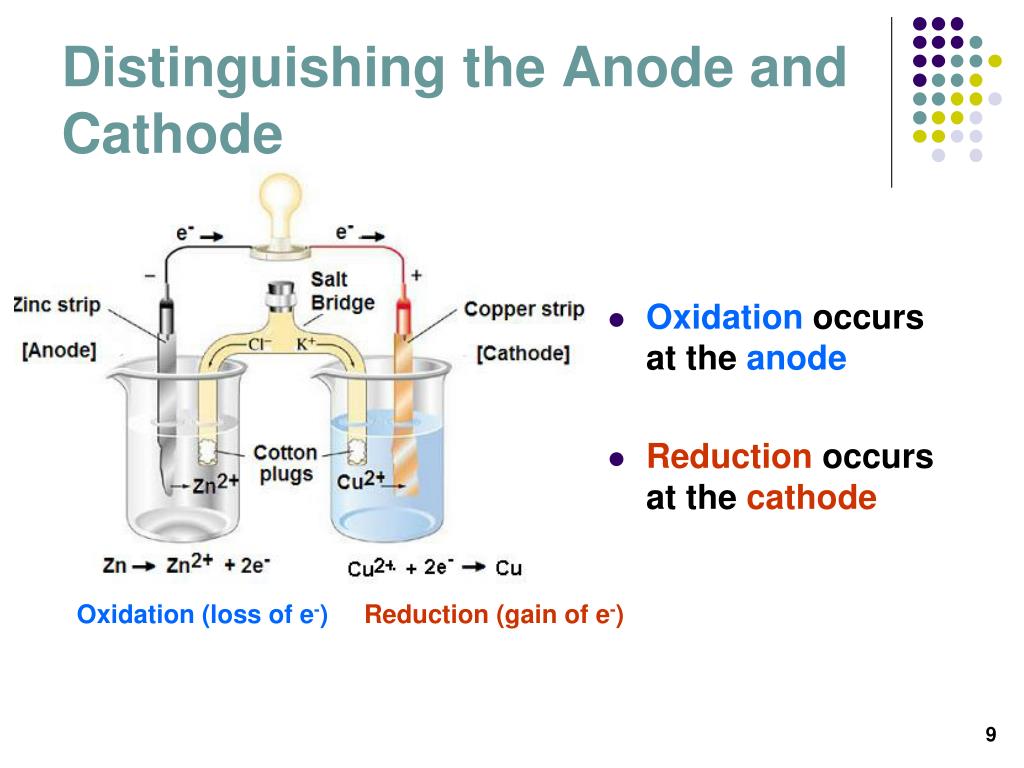
Therefore, the movement of electrons is opposite to that of the current flow. Electrons have a negative electrical charge. A conventional current shows the direction in which positive charges move. This can be recalled by using the mnemonic Cathode Current Departs. It is an electrode from which a current leaves a polarized electrical device. 2 FAQs on Cathode and Anode What is a Cathode?


 0 kommentar(er)
0 kommentar(er)
